Lab 6 – Bonding by BP, Solubility, and IR
We offer reliable assignment help.
Name: [Full and Last]
Date: MM/DD/YYY
Experiment #: 6
Title: Bonding by BP, Solubility, & IR
Purpose:
This experiment aims to evaluate the water solubility, boiling temperatures, and infrared spectra of various organic compounds to explore their intermolecular and intramolecular bonding. In this analysis, students will discover how to identify and identify between ionic, polar, nonpolar, and hydrogen-bonding substances using physical attributes and spectroscopic data.
Procedure:
- Solubility tests
- Put a little amount of water in the test tube
- Add a small amount of the sample.
- Shake to mix the sample with water
- Observe the change, whether the sample dissolves in water
- Boiling point
- Put a sample of up to half of the flask
- Place the flask on the stand above the burner
- Dip the temperature probe into the flask for temperature readings
- The temperature reading when the vapour is produced is the boiling of the sample
- IR
- Place the sample into the IR
- Set the resolution to 4.00cm
- Close the IR
- Turn it on and observe the spectrum of the sample on the monitor
Data/Results/Calculations:
- Standard Boiling points
| Compound | Boiling points | Dipole moment
(relative polarity, D) |
| Potassium chloride | 1420 o C | 10.2 |
| Ethyl alcohol | 76.4 o C | 1.7 |
| Acetic Acid | 116.2 o C | 1.5 |
| n-butyl alcohol | 115.3 o C | 1.7 |
| t-butyl alcohol | 81.5 o C | 1.7 |
| n-propyl chloride | 46.3 o C | 2.1 |
| n-pentane | 34.8 o C | 0 |
Molecular weights
| Compound | Molecular weight | Dipole moment (D) |
| Potassium chloride | 74.5 g/mol | 10.2 |
| Ethyl alcohol acetic acid | 46 g/mol | 1.7 |
| n-butyl alcohol | 60 g/mol | 1.5 |
| t-butyl alcohol | 74 g/mol | 1.7 |
| n-propyl chloride | 74 g/mol | 2.1 |
| n-pentane | 72 g/mol | 0 |
Infrared Spectroscopy (IR)
A spectrometer is a device that transmits an infrared light beam across a sample, causing the hydrogen bonds of the sample molecules to bend or stretch as the infrared light of specific wavelengths is absorbed.
A graph (spectrum) of the light that passes through is created, with inverted heights corresponding to energy absorption by the bonds. The arrangement of peaks in this spectrum tells what bonds are present and how they are ordered inside the sample molecules.
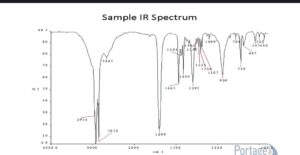
Sample IR spectrum
IR Spectrum of Substances
- Acetic acid
Acetic acid’s infrared spectrum reveals a very high and broad signal for the O-H extending around 3400-2500 cm1. The elongation of the C=O bond has a high abrupt peak at 1700 cm1. The winding of the C-H bonds in the methyl group has a peak in the 1500-1400 cm1 area. The C-O bond stretches between 1300 and 1200 cm 1. As a result of the IR spectrum, Acetic acid comprises solely intramolecular in C-H, C-C, C=O, O-H, and C-O bonds.
- t-Butyl alcohol
The infrared spectra of t-Butyl ethanol reveal the highest and broadest peak for the O-H extend at 3500-3200 cm1. There are multiple peaks in the 3000-2800 cm1 range for C-H bond stretching. Additional peaks for the bend in the bonds between C and H in the group methyl can be found in the 1500-1300 cm1 range. The stretching of the C-O bond takes place around 1200 cm 1. As a result of the IR spectrum, t-butyl alcohol comprises exclusively intramolecular in C-H, C-C, O-H, and C-O bonds.
- n-Butyl alcohol
The infrared spectra of n-Butyl alcohol demonstrate two significant peaks for the O-H stretch at 3700-3600 cm1 and 3400-3300 cm1. There are multiple peaks in the 3000-2800 cm21 range for C-H bond stretching. There are more peak values in the 1500-1300 cm1 range for C-H bond bending in the methyl and ethyl groups. The C-O bond begins to stretch around 1050 cm 1. According to the IR spectra, n-Butyl alcohol is exclusively comprised of intramolecular C-H, C-C, O-H, and C-O bonds.
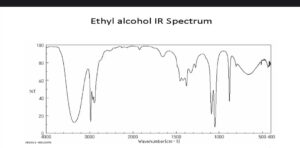
- Ethyl alcohol
The infrared spectra of the ethyl alcohol indicate the highest high and wide crest for the O-H stretch, around 3400-3200 cm1. There are multiple peaks in the 3000-2800 cm1 range for C-H bond stretching. There are further peak values in the 1500-1300 cm1 range for C-H bond bending in the CH3 and CH2 groups. The stretching of the C-O bond takes place at 1050 cm3 As a result of the IR spectrum, Ethyl alcohol has exclusively intramolecular C-H, C-C, O-H, and C-O bonds, just like n-Propyl chloride.
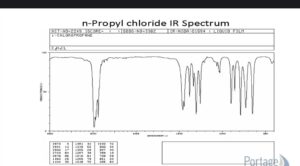
- n-Propyl chloride
The extension of the C-H bonds is seen in the infrared spectra of n-Propyl chloride, with many peaks in the 3000-2800 cm1 area. There are further peaks at 1460 cm 1 and 1380 cm 1 for C-H bond bending in the CH3 and CH2 groups. The absorption maxima for the C-Cl bond are 730 cm 1 and 650 cm 31. As a result of the IR spectrum, n-Propyl chloride comprises exclusively intramolecular C-H, C-C, and C-Cl bonds.
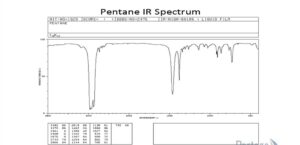
- Pentane
Pentane’s infrared spectrum includes many crests between 3000-2800 centimetre -1 ranges for C-H bond elongating. There are more points between 1460 centimeter-1 and 1380 centimeter-1 for C-H bond bending in the CH3 and CH2 groups. As a result of the IR spectrum, Pentane has only intramolecular C-H and C-C bonds.
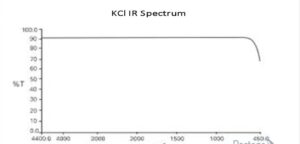
- KCI
KCI’s infrared spectrum is transparent throughout the whole IR range, without absorption points as the K-CI bond does not absorb radiation.
Boiling point
KCI has the strongest inter-molecular interactions hence a high boiling point (1420°C), as expected. The entire nonpolar chemical (n-pentane, CH2CH2CH2CH2CH3) possesses the smallest boiling point (36°C) due to its softest inter-molecular boding.
n-Propyl chloride (CH3CH2CH2CI) with (47°C). Comes second because of its weaker inter-molecular interactions after KCl,
The pattern of boiling point among the H-bonding requires some description to clearly distinguish them, despite the fact that the compounds have the 2nd-greatest figure of boiling temperatures, as expected given their second largest inter-molecular bonds.
Because of its greater molecular weight of 74, n-butyl alcohol has a higher boiling point of 115oC than ethyl alcohol and highly branched t-butyl alcohol, which have lower molecular weights.
But why is the boiling temperature of acetic acid (116.2 C) equal to that of n-butyl alcohol when acetic acid has a mass of 60 and n-butyl alcohol has a mass of 74?
The compound acetic acid has an extraordinarily high boiling point because it can form two H connections between two molecules, whereas all other Hydrogen bonds between molecules (such as n-butyl ethanol) are limited to creating one. Because of the increased degree of hydrogen bonding, acetic acid has an unexpectedly higher boiling point.
Effect of Branching (Symmetry)
A larger degree of branches causes molecules with approximately identical intermolecular interactions and molecular weights to have better symmetry. Increased symmetrical leads to a smaller area on the surface, which requires low force to split the atoms that form separate gaseous forms; hence compounds that have more branching will have lower boiling temperatures.
An ionic compound (such as potassium chloride, in the preceding list) ought to possess the greatest boiling temperatures because it possesses the most powerful intermolecular interactions.
Because it has the least intermolecular interactions, a totally nonpolar chemical (such as n-pentane) ought to have the least boiling temperatures. A polar (but non-H-bonding) element (such as n-Propyl chloride) has the lowest boiling point, considering it has the second-weakest inter-molecular connections.
Because they have the highest inter-molecular forces, H-bonding chemicals (such as the acid acetic acid, ethanol ethyl, n-butyl alcohol, and t-butyl alcohol) should have the second biggest boiling temperature set.
Effect of Molecular Weight
The greater the molecular mass among compounds with roughly comparable intermolecular interactions, the larger the molecules’ mass and surface area. Greater surface area results in greater bonds between heavier molecules, needing more energy to separate the molecules into separate gas particles, leading to higher boiling temperatures for molecules with higher molecular weights.
Effect of Inter-molecular forces
The boiling points of compounds are largely influenced by the intermolecular interactions that hold molecules together in aggregates, causing them to exist in liquid form. The higher the boiling temperatures of the components with greater interactions between molecules, the more energy is required to separate the masses into single gas particles. The relative intensity of intermolecular forces is as follows:
Ionic bond > H-bonding > D-dipole > Weak Van der Waals forces
Solubility Study
The ability of compounds to dissolve in water, a polar H-bonding and solvent, can suggest inter-molecular bonding. In general, ionic, polar, or hydrogen-bonding compounds will dissolve in water. Molecules that possess no polar bonds or a high hydrogen and carbon content cannot dissolve in water.
KCI dissolved in water because it has ionic bonds
Water solubility was demonstrated for ethanol, t-butyl alcohol, and acetic acid, suggesting that they are Hydrogen-bonding molecules.
n-Butyl alcohol appeared to be partly soluble, showing that the nonpolar 4-carbon chain reduced its solubility despite its Hydrogen bonding feature.
Given the nonpolar 3 or 5 chains of carbon in their molecules, Pentane and n-propyl chloride are insoluble.
Pentane and n-Propyl chloride solubility was investigated further, and it was discovered that they are soluble in one another, showing that they are nonpolar compounds (insoluble in water because it is polar) but soluble in one another (both nonpolar).
Conclusion:
In conclusion, through this experiment, students investigated the intermolecular and intramolecular bonding of various organic compounds by comparing their water solubility, boiling points, and Infrared spectra. By analyzing the physical properties and spectroscopic data, students learned to identify and differentiate between ionic, polar, nonpolar, and hydrogen-bonding compounds. The purpose of the experiment was successfully achieved, providing valuable knowledge and understanding of organic compound characteristics and bonding types.
Notes:
Nonpolar compounds dissolve in nonpolar solvents due to their similar molecular interactions, where weak Van der Waals forces attract and disperse the compound. Polar compounds dissolve in polar solvents due to their ability to form intermolecular hydrogen bonds or dipole-dipole interactions, promoting solubility through stronger attractive forces.
ORDER A PLAGIARISM-FREE PAPER HERE
We’ll write everything from scratch
Question

Lab 6 – Bonding by BP, Solubility, and IR
This lab will be conducted by an experienced instructor. As you watch the lab, you should keep a lab notebook just as you would if you were personally conducting the lab. A well-kept lab notebook is the key to successful labs. The lab notebook acts as the record of your experiment, help you organize your thoughts and understand the results of the experiment and will be useful in writing your lab report. Your lab notebook and/or lab report can be used as you take the lab exam that accompanies each experiment.
To complete the lab report requirement, be sure to follow the “Lab Report Sample” as your model and type out your lab report using the “Lab Report Template.” These can be found under “Lab Overview”. You can upload your report by clicking “Submit,” and then attaching your document.