Lab 5 – Gas Laws
Name: [Full and Last]
Date: MM/DD/YYY
Experiment #: 5
Title: Gas Laws
Purpose:
The primary purpose of this lab is to investigate the Ideal Gas Law, Boyle’s Law, and Charles’ Law. The experiments in this lab also aim to help one understand the relationships between pressure and volume, as well as volume and temperature in gases. Additionally, the experiment will predict the molar mass of a gas utilizing the Ideal Gas Law.
Procedure:
- Boyle’s Law
- A syringe plunge is used to measure the volume of the gas.
- Press the plunge to add more pressure on the gas inside the syringe.
- Note: The gas gets compressed as the plunge pushes it down.
- Add more pressure by pressing the plunger while measuring the volume of the gas at each interval.
- Charles’ Gas Law
- Suspend the flask on the stand.
- Fix the special sucker (syringe) at the top of the flask.
- Dip the thermometer into the flask.
- Measure the initial temperature in the flask.
- Heat the flask while observing the top and the bottom of the syringe plunger while heating the flask.
- Record the temperature after every 5 minutes (take the reading from the thermometer).
- Ideal Gas Law
- Cover the flask using the foil.
- Put the flask on the balance to determine the mass (Initial Mass).
- Measure the mass of acetone and put it in the flask.
- Cover the flask.
- Put the flask in the water bath.
- Dip the thermometer in the water bath.
- Heat the water using the Bunsen burner.
- To ensure all acetone has been vaporized, boil the water up to 66oC, 10oC above the boiling point of acetone (56oC).
- After heating, take the flask out of the water bath and allow it to condense any acetone in vapor form.
- Put the flask on the balance to measure the liquid acetone.
- Calcium Carbonate Molar Mass Determination
- Put 2 ml of HCl in the syringe.
- Measure 0.2152 grams of CaCO3 and put it in the test tube.
- Suspend the test tube containing CaCO3 in the stand.
- Fit the syringe at the top of the test tube.
- Fix the gas collecting tube at the top of the test tube.
- Measure the initial volume of gas before the reaction.
- Press the syringe plunger to allow HCl to drop into the test tube for reaction.
- After the reaction, measure the final volume of gas (Gas Produced).
Data/Results/Calculations:
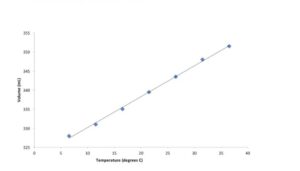
Charles’ Gas Law Graph
Boyle’s Law Graph
Ideal Gas Law
PV = nRT
Standard Temperature and Pressure (STP) = 0.0oC (273.15K) 1atm
V = 1.0mole × 0.0826 L–atm/mol × 273K/1.0atm = 22.4 L
- Acetone Molar Mass Determination
Mm = mRT/PV
m = 0.6527g
T = 66.99 o C
P = 0.9766 atm
V = 328ml (0.328L)
Mm = 0.6527g × 0.08206 L–atm/mol × 339.6 K/ 0.9766 atm × 0.328L = 56.8g/mol
Molar mass of Acetone = 58.08g/mol
Error = 58.08g/mol – 56.8g/mol/58.08g/mol × 100 = 2.2%
- Calcium Carbonate Molar Mass Determination
n = Pv/RT
= 0.9766atm × 0.048ml /0.08206 L–atm/mol X 295.7
n = 0.00196 mol CO2
1 mol CO2 = 1 mol CaCO3
1 mol CaCO3 = 0.00196mol × 100g/1mol = 0.196CaCO3
Error = 0.2152g – 0.196g/0.2152g × 100 = 8.9%
Conclusion:
In conclusion, the purpose of this lab was successfully achieved. By conducting experiments, Boyle’s Law and Charles’ Law were investigated, observing the inverse relationship between volume and pressure and the direct relationship between temperature and volume in gases. The experiment predicted the molar mass of a gas utilizing the Ideal Gas Law.
Notes:
Gas laws play a vital role in everyday life activities. Notably, understanding concepts like Ideal Gas Law, Boyle’s Law, and Charles’ Law helps in comprehending phenomena such as breathing, weather patterns, combustion, and gas storage. Applications include respiratory health, climate science, energy production, and transportation systems.
ORDER A PLAGIARISM-FREE PAPER HERE
We’ll write everything from scratch
Question

Lab 5 – Gas Laws
This lab will be conducted by an experienced instructor. As you watch the lab, you should keep a lab notebook just as you would if you were personally conducting the lab. A well-kept lab notebook is the key to successful labs. The lab notebook acts as the record of your experiment, helps you organize your thoughts and understand the experiment’s results and will be useful in writing your lab report. Your lab notebook and/or lab report can be used as you take the lab exam accompanying each experiment.
To complete the lab report requirement, be sure to follow the “Lab Report Sample” as your model and type out your lab report using the “Lab Report Template.” These can be found under “Lab Overview”. You can upload your report by clicking “Submit” and then attaching your document.