Discussion – Patient Medication Guide
Description of Aripiprazole
Aripiprazole, known by its brand names Abilify, Abilify Maintena, and Aristada, is an atypical antipsychotic medication primarily indicated for the treatment of schizophrenia in adults and adolescents (13-17 years), bipolar I disorder (both as monotherapy and as an adjunct therapy in adults and children aged 10-17), adjunctive treatment of the major depressive disorder (MDD) in adults, irritability associated with autistic disorder in pediatric patients (aged 6-17 years), and treatment of Tourette’s disorder in pediatric patients (aged 6-18 years) as indicated by Gettu and Saadabadi (2022). Additionally, it has been used off-label for conditions such as borderline personality disorder and behavioral disturbances in dementia.
Drug Classification
Aripiprazole belongs to the group of second-generation antipsychotics.
Mechanism of Action
From the findings made by Gettu and Saadabadi (2022), aripiprazole acts as a D2 partial agonist, 5-HT1A receptor agonist, and an antagonist to the 5-HT2A receptor. It helps in moderating the amount of Dopamine and Serotonin neurotransmitters in the brain which is believed to help manage psychosis and mood disorders.
Pharmacokinetics
Aripiprazole has an oral bioavailability of approximately 87% according to the study, aripiprazole can be distributed in all the body tissues including the human body, and is mainly metabolized in the liver with the help of CYP2D6 and CYP3A4 enzymes. It has a biotransformation of 75 hours to 146 hours and is excreted in urine and feces.
Pharmacodynamics
Aripiprazole can act as a partial agonist of D2 and 5-HT1A receptors together with the antagonist of the 5-HT2A receptors that are involved in the regulation of neurotransmission in the brain areas related to psychosis and mood disorders (Gettu & Saadabadi, 2022).
Dosing and Administration
In adults with schizophrenia, dosing varies between 10-15 mg initially in the first 24 hours and a maintenance dosage of 10-30 mg per 24 hours. In bipolar I, the initial starting dose is 15 mg/ day and the maintenance dose range is 15-30 mg/ day. In the adjunctive treatment of MDD, the initial dose is 2- 5 mg per day with a recommended daily dose of 5- 10 mg (Weiden et al., 2020). Aripiprazole is available in many formulations such as oral tablets, orally disintegrating tablets, oral solutions, and intramuscular injections of the extended-release formulation.
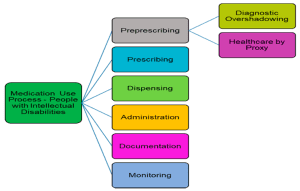
Specialty Population Considerations
Before administering aripiprazole to some patient populations, precautions are required. Children and adolescents require dosing based on the clinical effect and toxicity. Extra care should be taken when prescribing drugs to elderly patients since they are vulnerable to the effects of drugs, besides may have a slow metabolism. The decision to use aripiprazole during pregnancy should be made only if the potential benefits of administering this medicine are significantly higher than the possible risks. More attention should be paid to those patients who have suicidal behaviors since the risk of suicidal ideation related to the use of antipsychotics may rise.
Half-Life
Half-life is also a very significant concept in pharmacology because it determines the dosing interval and also gives information about the build-up and the equilibrium concentration of the drug. It has a half-life of 75 hours for oral and 146 hours for long-acting injection as mentioned by Correll et al. (2021).
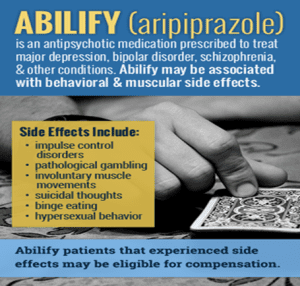
Side Effects and Adverse Reactions
Common side effects of aripiprazole have been reported to include nausea, vomiting, constipation, headache, dizziness, anxiety, and insomnia (Torrico et al., 2020). The most serious side effects include EPS, tardive dyskinesia, NMS, alterations in glucose and lipid profile, and postural hypotension. EPS consists of involuntary movements of the extremities and the face and may mandate a reduction in the dosage or discontinuation of the medication. Tardive dyskinesia which is characterized by persistent involuntary movements should be assessed using rating scales such as AIMS.
Contraindications
Aripiprazole should not be given to patients who have an allergic reaction to the drug or any of the ingredients or to patients with severe hepatic impairment because of its capacity to accumulate in the body and become toxic (Gettu & Saadabadi, 2022).
Overdose Considerations
Overdose symptoms may include vomiting, somnolence, tremors, and EPS and management is supportive, with attention to the airway, hydration, and cardiovascular status.
Diagnostics and Labs Monitoring
It is recommended that patients who received aripiprazole should have initial and subsequent assessments, including weight, fasting blood glucose, lipid profiles, and complete blood counts (CBC) (Gettu & Saadabadi, 2022). Blood sugar levels should be checked at least once a day in diabetic patients; in the case of dyslipidemia, lipid levels should be monitored.
Legal, Ethical, and Social Considerations
Legal, ethical, and social issues in the use of antipsychotics include regulatory prescriptive requirements and monitoring, consent, and issues of stigmatization, especially where the drug is used off-label or in children and adolescents.
Patient Education
It is crucial to educate the patient when prescribing aripiprazole. Patients should be educated on side effects, compliance with said schedule, signs of severe reactions, and when to seek medical attention. Also, the necessity of additional lab tests and follow-up appointments should be discussed.
References
Correll, C. U., Kim, E., Sliwa, J. K., Hamm, W., Gopal, S., Mathews, M., Venkatasubramanian, R., & Saklad, S. R. (2021). Pharmacokinetic Characteristics of Long-Acting Injectable Antipsychotics for Schizophrenia: An Overview. CNS Drugs, 35(1), 39–59. https://doi.org/10.1007/s40263-020-00779-5
Gettu, N., & Saadabadi, A. (2022). Aripiprazole. PubMed; StatPearls Publishing. https://pubmed.ncbi.nlm.nih.gov/31613519/
Torrico, T., Kiai, N., Meza, C., Salam, M. T., & Abdijadid, S. (2020). Suspected Aripiprazole-induced neutropenia in a geriatric patient: a case report. BMC Geriatrics, 20(1). https://doi.org/10.1186/s12877-020-01514-x
Weiden, P. J., Du, Y., von Moltke, L., Wehr, A., Hard, M., Marandi, M., & Walling, D. P. (2020). Pharmacokinetics, Safety, and Tolerability of a 2-Month Dose Interval Regimen of the Long-Acting Injectable Antipsychotic Aripiprazole Lauroxil: Results From a 44-Week Phase I Study. CNS Drugs, 34(9), 961–972. https://doi.org/10.1007/s40263-020-00745-1
ORDER A PLAGIARISM-FREE PAPER HERE
We’ll write everything from scratch
Question
This week, you will create a Medication Study Guide to share with your peers. This guide is intended to be a useful learning tool for you to use as you prepare for your clinical courses.
Patient Medication Guide
You will be assigned one of the following medications to create your guide:
Chlorpromazine
Fluphenazine
Haloperidol
Loxapine
Perphenazine
Aripiprazole
Asenapine
Clozapine
Iloperidone
Olanzapine
Paliperidone
Quetiapine
Risperidone
Ziprasidone
Lurasidone
Brexpiprazole
Cariprazine
Lumateperone
Benztropine
Propranolol
Deutetrabenazine
Valbenazine
RESOURCES
Be sure to review the Learning Resources before completing this activity.
Click the weekly resources link to access the resources.
WEEKLY RESOURCES
TO PREPARE FOR THIS ASSIGNMENT:
Identify your assigned psychotropic medication agent.
Review this week’s Learning Resources, including the medication resources indicated for this week.
Reflect on the psychopharmacologic treatments you might recommend for the assessment and treatment of vulnerable patient populations requiring antidepressant therapy.
THE ASSIGNMENT
Create a 3- to 4-page (excluding visual elements) Medication Study Guide for your assigned psychotropic medication agents that may be utilized by you and colleagues for study. Your medication guide should be in the form of an outline and should include a title page, citations, and references. You should incorporate visual elements, such as concept maps, charts, diagrams, images, color coding, mnemonics, and/or flashcards. Be creative!
Note: Your Medication Study Guide should not be in the format of an APA paper.
Also note: Your guide should be informed by the FDA-approved and Evidence-Based Clinical Practice Guidelines Research.
Areas of importance that you should address—but are not limited to—include:
Title page
Description of the psychopharmacological medication agent, including brand and generic names, as well as appropriate FDA-approved indications and uses
Any supporting, valid, and reliable research for non-FDA uses
Drug classification
The medication’s mechanism of action
The medication pharmacokinetics
The medication pharmacodynamics
Appropriate dosing, administration route, and any considerations for dosing alterations
Considerations of use and dosing in specific specialty populations, such as children, adolescents, the elderly, pregnant people, those exhibiting suicidal behaviors, etc.
Definition of half-life, why half-life is important, and the half-life for your assigned medication
Side effects/adverse reactions potential
Discuss clinical concerns with EPS and Tardive Dyskinesia
Note: Be sure to include screening tools that would be utilized.
Contraindications for use, including significant drug-to-drug interactions
Overdose considerations
Diagnostics and labs monitoring comorbidities considerations
Legal, ethical, and social considerations
Pertinent patient education considerations
References page
Support your rationale with a minimum of three (3) academic resources.