Chemistry Discussion-Converting Lbs to Kilograms and Naming Compounds
Question One: Converting Lbs to Kilograms
2240 lbs equals 1016.047 kilograms. I used the conversion formula for pounds to kilograms to determine the mass in kilograms, which is equivalent to 2240 lbs.
1lb = 0.4536Kgs
2240lbs =?
= (2240 × 0.4536) ÷ 1
=1016. 047Kgs
Therefore, 2240 lbs equals 1016.047 kilograms.
Question Two: Naming Compounds
NaF – Sodium fluoride
I used the binary naming system in chemistry to name the compound. The cation was named first before the non-metal element.
Rb2O – Rubidium oxide
I used the binary naming system in chemistry to name the compound. The cation was named first before the non-metal element. In this case, rubidium has a net charge of +1, while oxygen has a net charge of -2. This means that two atoms of rubidium are required to neutralize the charges on the oxygen atom.
BCl3 – Boron trichloride
I used the binary naming system in chemistry to name the compound. The cation was named first before the non-metal element. Boron has an oxidation state of +3, while chlorine has an oxidation state of -1. Three atoms of chlorine are required to neutralize the charge on the boron atom.
H2Se – Hydrogen selenide
I used the binary naming system in chemistry to name the compound. The cation was named first before the non-metal element.
P4O6 – Tetraphosphorus hexoxide
I used the binary nomenclature system for binary covalent compounds. The first element retains its neutral element name, while the second atom has the ending of its name replaced with -ide. A prefix is added to the name, denoting the number of atoms involved. In this case, tetra- denoted four atoms of phosphorous, and hexo- indicated six atoms of the oxygen atom.
ICl3 – Iodine trichloride
I used the binary naming system of two non-metals to name the compound. Iodine came first because it is less electronegative than chlorine. The prefix tri- was used to denote the number of chloride atoms involved. The first atom retained its neutral name, while the second had its ending replaced with a -ide.
ORDER A PLAGIARISM-FREE PAPER HERE
We’ll write everything from scratch
Question
Chemistry Discussion-Converting Lbs to Kilograms and Naming Compounds
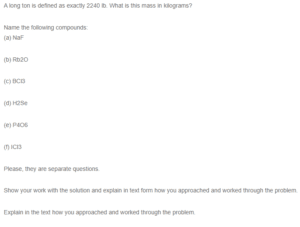
A long ton is defined as exactly 2240 lb. What is this mass in kilograms?
Name the following compounds:
(a) NaF
(b) Rb2O
(c) BCl3
(d) H2Se
(e) P4O6
(f) ICl3
Please, they are separate questions.
Show your work with the solution and explain in text form how you approached and worked through the problem.
Explain in the text how you approached and worked through the problem.

