An Investigation of Mole Ratios and Limiting Reactants
DETERMINING THE OPTIMUM MOLE RATIO FOR THE FORMATION OF CO2 FROM THE REACTION OF SODIUM BICARBONATE AND ACETIC ACID.
Objectives:
The purpose of the lab experimentation was to determine the maximum mole ratio needed for the reaction between sodium bicarbonate, which is basic, and acetic acid. The maximum mole ratio is reached when the reaction produces the highest amount of Carbon IV Oxide gas. This is why one of the guiding questions that the investigation aims to answer is the optimum mole ratio for the formation of Carbon IV Oxide from the reaction of sodium bicarbonate and acetic acid. This is an example of a neutralization reaction between a base and an acid. When a bicarbonate mixes with acid, it breaks down into CO2 and H2O. The task in the current experiment is to design and conduct an investigation to determine the optimum mole ratio required for complete neutralization. It involves comparing the amounts of CO2 produced when different amounts of sodium bicarbonate are reacted with different amounts of acetic acid. It was also crucial to identify the limiting reactant in other reactions.
Introduction/Overview/Background:
The reaction between an acid and a base is known as a neutralization reaction. The maximum amount of CO2 measures the optimal amount in moles of reactants required for the reaction. The process of determining the amount of reactants needed for the reaction or the product produced is known as stoichiometry. In the calculation of most stoichiometry reactions, most of the calculations are performed using the exact mole ratios of the reactants and products. For real-life commercial processes in the commercial preparation of compounds, these reactions may entail using an excess amount of one reactant, where the other reactant acts as the limiting agent since it limits the amount of the other reactant used up in the reaction (Neavyn, Boyer, Bird, & Babu, 2013). This indicates that if two reactants are not mixed in correct ratios, there is a high possibility that less product will be produced. In the current reaction, the gas is collected through the displacement of water.
Another way to predict the number of moles of a product produced in a chemical reaction can be derived from a well-balanced equation. In this case, once the equation is balanced, the moles of carbon (iv) oxide produced in the reaction. Once the moles are determined, the weight can be established. Sodium bicarbonate is also referred to as solid baking soda, while acetic acid is known as vinegar. Usually, different carbonates weigh differently, but when the same amount of reactants is used, a similar reaction occurs. Although the two chemicals are household chemicals and foodstuffs, it is advisable that the process be undertaken under care. No chemicals should be touched during experimentation.
Materials used:
1.00 M Acetic Acid (HC2H3O2)
Sodium Bicarbonate (NaHCO3)
40ml Beaker
100ml and 25ml Graduated cylinders
Electronic balance
Plastic tray
Ring Stands/Rings
Side-arm flask with tubing
Eye droppers
Procedure:
Measure different masses of sodium bicarbonate using the top loader balance
Measure 25ml of acetic acid to react with the different amounts of the bicarbonate. The acetic acid solution is placed in the flask before adding sodium bicarbonate.
Fill the plastic tray halfway with water
Place the reactants in the side-arm flask with tubing. The tubing directs the gas produced into the graduated cylinder while placing the end of the tube in the cylinder.
The flask is closed well using a plug so that no CO2 The reactants are shaken to aid the reaction.
Using a ring stand, hold up and upside down the graduated cylinder full of water. This allows the water in the graduated cylinder not to drain due to water molecules colliding on the water’s surface and the bottom. This creates enough pressure to prevent water from flowing out of the graduated cylinder.
Using the inverted graduated cylinder, measure the amount of carbon iv oxide produced from the reaction by reading the change in volume using the water level.
Record each amount of CO2 produced corresponding to each mass and mole of NaHCO3
The trials are repeated five times while recording the amount of gas produced in different ratios of the reactants, as shown in Table 1 below.
Precaution:
Acetic acid needs to be handled with care. Also, since pressure is build up during the reaction, wear goggles at all times to avoid spillage on eyes.
Results and Discussion:
| Trial | 1.00 M acetic acid (HC2H3O2) Volume | 1.00 M acetic acid (HC2H3O2) Moles | Mole Ratio
HC2H3O2:NaHCO3 |
NaHCO3 MW: 84g/mol Moles | NaHCO3 MW: 84g/mol Mass needed | Volume of CO2 produced |
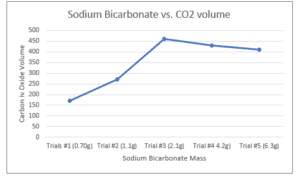
| 1 | 25.0ml | 0.025 L | 3:1 | 0.0083 L | 0.70 g | 170 ml |
| 2 | 25.0ml | 0.025 L | 2:1 | 0.0125 L | 1.1 g | 270 ml |
| 3 | 25.0ml | 0.025 L | 1:1 | 0.0250 L | 2.1 g | 460 ml |
| 4 | 25.0ml | 0.025 L | 1:2 | 0.0500 L | 4.2 g | 430 ml |
| 5 | 25.0ml | 0.025 L | 1:3 | 0.075 L | 6.3 g | 410 ml |
Figure 1: Sodium Bicarbonate Mass and CO2 Produced
Some visible fizzing reaction is experienced in the process, which subsides as the reactants get used up. This fizzing is produced due to the bubbles of carbon dioxide produced from the reaction. The two compounds react to form sodium acetate and carbonic acid, which rapidly decomposes into water and Carbon IV Oxide. This renders the reaction irreversible at room temperature and pressure conditions. The reaction involves both reactants dissociating in water, and the ions interchange the species. The two sodium cations leave the carbonate molecules and are attracted by the negative acetate charge and bond to it. Then the hydrogen cations are attracted to CO3 anions, which form a molecule of water, and CO2 molecule, which tends to join with other CO2 molecules to form CO2 bubbles in the solution that flees through the surface of the liquid. The reaction is as shown below;
Na2CO3 + 2 CH3COOH → 2 CH3COONa + CO2 + H2O
Conclusions:
The fact that no loss of mass is witnessed is proof that any carbonate reacts with an acid, and mass is conserved. In the reaction, the optimum mole ratio for the reactants is 1:1. For the first and second trials, sodium bicarbonate was the limiting agent, while in the third and fourth trials, acetic acid was the limiting agent. The worst yield in the five trials is in trial #1, where a lot more acetic acid than sodium bicarbonate causes faster fizzing than the other trials. The best results from the investigation were obtained from trial #3, where 460ml of carbon iv oxide was produced. This weight corresponds to the mole ratio of 1:1 of the reactants. After reaching this level, the reaction slowly takes off, stops increasing, and a downward trend is observed. Therefore, it takes 2.1g of sodium bicarbonate to completely react with acetic acid to produce an optimal amount of Carbon iv Oxide gas.
Post-Lab Questions
The concept being investigated and its relation to the guiding question
The concept being investigated was stoichiometry under a neutralization reaction. The concept relates to the guiding question since the question targets to investigate the optimum mole ratio for the formation of CO2 from the reaction of Sodium Bicarbonate and Acetic Acid.
The optimum mole ratio for the formation of CO2 from the reaction of Sodium Bicarbonate and Acetic Acid.
The mole ratio for optimum CO2 was 1:1. In this ratio, 2.1g of sodium bicarbonate is needed.
The pressure of oxygen gas produced at the atm?
2H2O → 2 H2O + O2
Hydrogen Peroxide Weight= 4.00g
At 500k
Volume = 250ml
PV= n x RT
P x 0.25 = 3x 0.0826x 500 K
P= 496.5atm
How many liters of CO2 are produced when 0.68 moles of ethane are at STP?
Weight= 0.68 x 4= 2.72g
V= (2.72 x 0.0826 x 310.15K)/ 1atm
V= 69L
How many liters of H2 will be required at a temperature of 300k and 3atm to consume 56 grams of N2?
V= (28.01 x 0.0826 x 573.15)/3atm
V= 442.02L
References
Neavyn, M. J., Boyer, E. W., Bird, S. B., & Babu, K. M. (2013). Sodium acetate as a replacement for sodium bicarbonate in medical toxicology: a review. Journal of medical toxicology: official journal of the American College of Medical Toxicology, 9(3), 250–254. https://doi.org/10.1007/s13181-013-0304-0
ORDER A PLAGIARISM-FREE PAPER HERE
We’ll write everything from scratch
Question
Prepare this lab report document with 2 line spacing in each paragraph.
Title
Objectives:
Write the main aim of this investigation.
Introduction/Overview/Background:
Apart from the lab manual, students must do more research on the topic related to the investigation and write the introduction part. You may also collect appropriate pictures and show them in this section if possible.
An investigation Of Mole Ratios and Limiting Reactants
Materials used:
It’s very important to record all materials used in the lab because the lab report must show what materials have been used in the experiment.
Procedure:
You may write the procedure in your own sentences based on your handle in the lab. If possible, you may also show some pictures dealing with the experiment.
Results and Discussion:
Show your results in the form of tables or plots (e.g., linear, exponential, or other correlation plots) and discuss your results for each section of the investigation.