Advanced Pharmacology and Pharmacotherapeutics
Drug Comparison Table
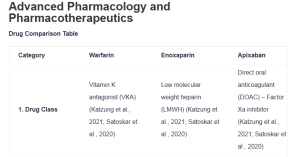
| Category | Warfarin | Enoxaparin | Apixaban | Clopidogrel |
| 1. Drug Class | Vitamin K antagonist (VKA) (Katzung et al., 2021; Satoskar et al., 2020) | Low molecular weight heparin (LMWH) (Katzung et al., 2021; Satoskar et al., 2020) | Direct oral anticoagulant (DOAC) – Factor Xa inhibitor (Katzung et al., 2021; Satoskar et al., 2020) | Antiplatelet agent – P2Y12 receptor antagonist (Katzung et al., 2021; Satoskar et al., 2020): Advanced Pharmacology and Pharmacotherapeutics |
| 2. Absorption | Well absorbed orally (>95%), peak levels 4 hours (Katzung et al., 2021; Satoskar et al., 2020) | Subcutaneous administration, bioavailability ~92%, peak anti-Xa activity 3-5 hours (Katzung et al., 2021; Satoskar et al., 2020) | Oral bioavailability ~50%, rapid absorption, peak plasma levels 3-4 hours (Katzung et al., 2021; Satoskar et al., 2020) | Oral absorption >50%, rapid absorption, peak plasma levels 1 hour (Katzung et al., 2021; Satoskar et al., 2020) |
| 3. Distribution | Highly protein bound (99%), a small volume of distribution (0.1-0.2 L/kg) (Satoskar et al., 2020) | Limited plasma protein binding (~20%), volume of distribution 4.3 L(Katzung et al., 2021) | Protein binding ~87%, volume of distribution ~21 L (Satoskar et al., 2020) | Protein binding ~98%, extensive tissue distribution (Katzung et al., 2021) |
| 4. Metabolism | Hepatic metabolism via CYP2C9, CYP1A2, and CYP3A4 enzymes (Katzung et al., 2021; Satoskar et al., 2020) | Minimal hepatic metabolism, primarily renal elimination of fragments (Katzung et al., 2021) | Hepatic metabolism via CYP3A4/5, biliary excretion ~25% (Katzung et al., 2021) | Hepatic metabolism via CYP2C19 to active metabolite, extensive first-pass effect (Katzung et al., 2021) |
| 5. Excretion | Renal elimination of metabolites (~92%), a small amount in feces (Katzung et al., 2021) | Renal excretion ~40% (active and inactive fragments), ~10% active fragments (Katzung et al., 2021) | Renal excretion ~27%, biliary/fecal ~25%, other pathways ~48% (Katzung et al., 2021) | Renal excretion ~50%, fecal ~46% (Katzung et al., 2021) |
| 6. Elimination Half-life | 20-60 hours (average 40 hours), varies significantly with age and genetics (Katzung et al., 2021) | 4.5-7 hours after subcutaneous injection, dose-independent (Katzung et al., 2021) | 8-15 hours, dose-independent (Katzung et al., 2021) | 6-8 hours (parent drug), active metabolite 30 minutes (Katzung et al., 2021) |
| 7. Indication | Atrial fibrillation, venous thromboembolism, mechanical heart valves, stroke prevention (Katzung et al., 2021) | DVT/PE treatment and prophylaxis, acute coronary syndromes, perioperative anticoagulation (Katzung et al., 2021) | Atrial fibrillation, venous thromboembolism treatment and prophylaxis, stroke prevention (Katzung et al., 2021) | Secondary prevention of atherothrombotic events, acute coronary syndromes, stroke prevention (Katzung et al., 2021) |
| 8. Side Effects | Skin necrosis, purple toe syndrome, bleeding (major concern), alopecia, teratogenicity (Katzung et al., 2021) | Thrombocytopenia, injection site reactions, bleeding, osteoporosis (long-term use) (Katzung et al., 2021) | Bleeding (lower major bleeding risk vs warfarin) (Steffel et al., 2021), nausea, hypersensitivity reactions (Katzung et al., 2021) | Dyspepsia, rash, thrombotic thrombocytopenic purpura (rare), bleeding (Katzung et al., 2021) |
| 9. Interactions | Extensive drug-drug interactions (CYP enzyme inducers/inhibitors), food interactions (vitamin K) (Katzung et al., 2021) | Minimal drug interactions, enhanced effect with antiplatelet agents (Katzung et al., 2021) | Moderate interactions with CYP3A4 and P-gp inhibitors/inducers, fewer interactions than warfarin (Katzung et al., 2021) | CYP2C19 inhibitors reduce efficacy; proton pump inhibitors may reduce effectiveness (Katzung et al., 2021) |
| 10. Contraindications | Pregnancy (except mechanical valves), severe liver disease, malignant hypertension, active bleeding (Katzung et al., 2021) | Thrombocytopenia, hypersensitivity to heparin, active major bleeding (Katzung et al., 2021) | Active pathological bleeding, severe hepatic impairment, hypersensitivity (Katzung et al., 2021) | Severe liver impairment (Eslami & Gheymati, 2020), hypersensitivity, active bleeding |
| 11. Efficacy | Effective for multiple indications, the gold standard for mechanical valves requires therapeutic monitoring (Katzung et al., 2021) | Highly effective for VTE prevention/treatment, predictable anticoagulant effect (Stevens et al., 2021) | Non-inferior to warfarin for stroke prevention in AF, superior efficacy in some VTE studies (Katzung et al., 2021) | Effective for secondary prevention of cardiovascular events, established efficacy in ACS (Collet et al., 2020) |
| 12. Safety Issues | High bleeding risk, narrow therapeutic window, teratogenic, drug and food interactions (Katzung et al., 2021) | Generally safe profile, lower risk of HIT compared to UFH, reversible with protamine (Katzung et al., 2021) | Lower major bleeding risk compared to warfarin (Burnett et al., 2016; Chen et al., 2020), no routine monitoring required | Generally well tolerated, bleeding risk lower than anticoagulants, rare but serious thrombotic events (Katzung et al., 2021) |
| 13. Monitoring Parameters | INR monitoring required (target 2.0-3.0 for most indications), CBC, liver function tests (Katzung et al., 2021) | Anti-Xa levels in special populations (obesity, renal impairment, pregnancy), CBC, creatinine (Katzung et al., 2021) | No routine monitoring is required; CBC and renal function baseline and periodic assessment (Katzung et al., 2021) | No routine monitoring is required; CBC baseline, watch for bleeding signs (Katzung et al., 2021) |
| 14. EBP Guideline/Study | 2019 AHA/ACC/HRS Atrial Fibrillation Guidelines recommend warfarin as an alternative to DOACs (Steffel et al., 2021); CHEST 2021 VTE Guidelines | CHEST 2021 Antithrombotic Therapy Guidelines recommend LMWH for acute VTE treatment (Stevens et al., 2021); ACCP Guidelines for perioperative management | 2019 AHA/ACC/HRS Guidelines recommend apixaban as the first line for stroke prevention in AF; AMPLIFY trial demonstrated non-inferiority for VTE treatment (Burnett et al., 2016; Chen et al., 2020) | 2020 ESC Guidelines for ACS recommend dual antiplatelet therapy; the CURE trial established efficacy in ACS prevention (Collet et al., 2020) |
References
Burnett, A. E., Mahan, C. E., Vazquez, S. R., Oertel, L. B., Garcia, D. A., & Ansell, J. (2016). Guidance for the practical management of the direct oral anticoagulants (DOACs) in VTE treatment. Journal of Thrombosis and Thrombolysis, 41(1), 206–232. https://doi.org/10.1007/s11239-015-1310-7
Chen, A., Stecker, E., & Warden, B. A. (2020). Direct oral anticoagulant use: A practical guide to common clinical challenges. Journal of the American Heart Association, 9(13). https://doi.org/10.1161/jaha.120.017559
Collet, J., Thiele, H., Barbato, E., Barthélémy, O., Bauersachs, J., Bhatt, D. L., Dendale, P., Dorobantu, M., Edvardsen, T., Folliguet, T., Gale, C. P., Gilard, M., Jobs, A., Jüni, P., Lambrinou, E., Lewis, B. S., Mehilli, J., Meliga, E., Merkely, B., . . . Siontis, G. C. M. (2020). 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). European Heart Journal, 42(14), 1289–1367. https://doi.org/10.1093/eurheartj/ehaa575
Eslami, V., & Gheymati, A. (2020). Clopidogrel‐induced liver damage: A case report and review of the literature. Clinical Case Reports, 8(12), 3023–3026. https://doi.org/10.1002/ccr3.3324
Katzung, B. G., Kruidering-Hall, M., Tuan, R. L., Vanderah, T. W., & Trevor, A. J. (2021). Katzung & Trevor’s pharmacology examination and board review (13th ed.). McGraw Hill Professional.
Satoskar, R. S., Rege, N., & Bhandarkar, S. D. (2020). Pharmacology and pharmacotherapeutics (26th ed.). Elsevier India.
Steffel, J., Collins, R., Antz, M., Cornu, P., Desteghe, L., Haeusler, K. G., Oldgren, J., Reinecke, H., Roldan-Schilling, V., Rowell, N., Sinnaeve, P., Vanassche, T., Potpara, T., Camm, A. J., Heidbüchel, H., Lip, G. Y. H., Deneke, T., Dagres, N., Boriani, G., . . . Field, M. (2021). 2021 European Heart Rhythm Association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. EP Europace, 23(10), 1612–1676. https://doi.org/10.1093/europace/euab065
Stevens, S. M., Woller, S. C., Kreuziger, L. B., Bounameaux, H., Doerschug, K., Geersing, G., Huisman, M. V., Kearon, C., King, C. S., Knighton, A. J., Lake, E., Murin, S., Vintch, J. R., Wells, P. S., & Moores, L. K. (2021). Antithrombotic therapy for VTE disease. CHEST Journal, 160(6), e545–e608. https://doi.org/10.1016/j.chest.2021.07.055
ORDER A PLAGIARISM-FREE PAPER HERE
We’ll write everything from scratch
Question 
Kinetics and Dynamics
Absorption, Distribution, Metabolism, Excretion, Receptor Activity
For this assignment you will create a table using Word. Compare and contrast 4 specific drugs used in thromboembolic disorders. Place the title for the table at the top. Place the drug names in the horizontal row at or near the top of the table and the content identified vertically by topic in the first left hand column.
Briefly complete your table by adding the content for the following 4 drugs.
- Warfarin
- Enoxaparin
- Apixaban
- Clopidogrel
You will investigate the drug by identifying the following per each row under the corresponding drug. There are 14 rows under the horizontal Drug Names.
Advanced Pharmacology and Pharmacotherapeutics
- 1. drug class 2. absorption 3. distribution, 4. metabolism 5. excretion 6. elimination 7. indication 8. side effects 9. interactions 10. contraindications 11. efficacy 12. safety issues- known adverse drug issues 13. monitoring parameters 14. Identification of an evidence-based practice indication guideline/ study for each, that includes the specific pharmaceutical use identified within the guideline.
The table should be usable, neat and meaningful for content and visually sensible.Do Not use Excel. Create a table in Word.
- A title page is present
- The table is visually appropriate in the document and has sufficient margins
- A reference page is present
- I’ve attached a blank example table